A pH meter is an electronic instrument used to measure the acidity or alkalinity of a solution, expressed as pH. The pH scale ranges from 0 to 14, where a pH of 7 indicates neutrality, values below 7 indicate acidity, and values above 7 indicate alkalinity. A pH meter provides precise pH readings, which are essential in various scientific, industrial, and environmental applications.
Construction of a pH Meter
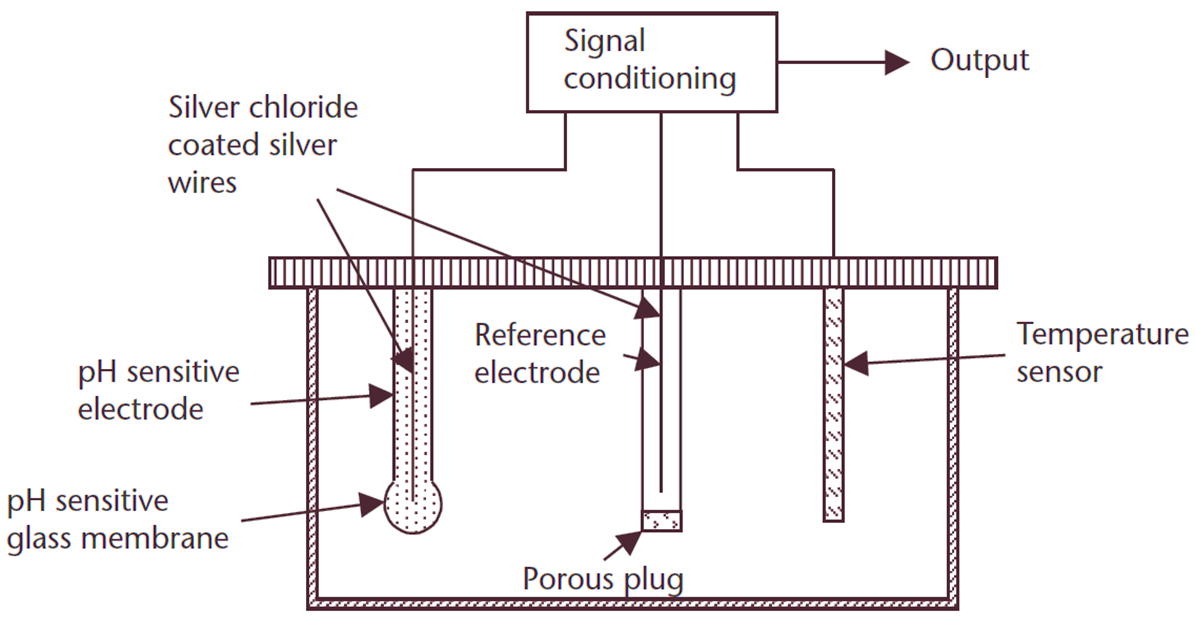
A pH meter typically consists of the following components:
- pH-Sensitive Electrode: The main sensor of the pH meter, usually made of glass, which responds to hydrogen ion activity. It has a pH-sensitive glass membrane at its tip that interacts with the solution being tested.
- Reference Electrode: Contains a stable electrolyte solution (commonly potassium chloride) and serves as a reference point for the pH measurement. Includes a porous plug for ionic exchange with the test solution.
- Silver Chloride Coated Silver Wires: Present inside both the pH-sensitive and reference electrodes for electrical connectivity.
- Temperature Sensor: Measures the temperature of the solution since pH measurements are temperature-dependent.
- Signal Conditioning and Output: Converts the small voltage generated by the electrodes into a readable pH value. The output is displayed on a digital or analog screen.
- Electrolyte Solution: Maintains the ionic balance inside the electrodes to facilitate accurate readings.
Working Principle of a pH Meter
The working of a pH meter is based on the Nernst equation, which relates the electric potential generated by the pH-sensitive electrode to the hydrogen ion concentration in the solution.
- Electrode Interaction: The pH-sensitive electrode interacts with hydrogen ions in the test solution. The reference electrode provides a stable voltage as a comparison point.
- Voltage Generation: The difference in hydrogen ion concentration between the inner solution of the electrode and the external solution generates a small voltage.
- Temperature Compensation: The temperature sensor adjusts the pH reading to account for the effect of temperature on the ion activity.
- Signal Processing: The generated voltage is processed by the signal conditioning unit, which converts it into a pH value displayed on the meter.
Types of pH Meters
| Type | Description |
|---|---|
| Benchtop pH Meters | Used in laboratories for precise measurements with advanced features like data logging. |
| Portable pH Meters | Lightweight and battery-powered, ideal for fieldwork and on-site testing. |
| Pen-Type pH Meters | Compact and easy to use, suitable for quick tests in less demanding applications. |
| Industrial pH Meters | Designed for continuous monitoring in industrial processes, often integrated with automation systems. |
| Specialized pH Meters | Tailored for specific applications, such as food, pharmaceuticals, or soil testing. |
Advantages of pH Meters
- High Precision: Offers accurate measurements with minimal errors.
- User-Friendly: Easy to operate with digital displays and automatic calibration features.
- Versatility: Applicable across various fields, including environmental science, biology, and industry.
- Portability: Portable models allow for on-site testing.
- Wide Range of Measurements: Suitable for acidic, neutral, and basic solutions.
Disadvantages of pH Meters
- Calibration Requirement: Requires frequent calibration for accurate results.
- Fragility: The glass electrode is delicate and prone to breakage.
- Dependency on Temperature: Measurements can be affected by temperature changes if not compensated.
- Maintenance: Electrodes need to be cleaned and stored properly to prevent contamination or damage.
- Cost: High-quality pH meters can be expensive.
Applications of pH Meters
- Environmental Monitoring: Testing the pH of water bodies to monitor pollution levels and aquatic health.
- Agriculture: Soil pH testing to optimize crop growth and fertilizer application.
- Food and Beverage Industry: Ensuring product quality by monitoring the pH of ingredients and final products.
- Pharmaceuticals: Measuring the pH of solutions in drug formulations and quality control.
- Chemical Industry: Monitoring reactions and ensuring the safety of chemical processes.
- Wastewater Treatment: Controlling pH during the treatment process to comply with environmental regulations.
- Biotechnology and Research: Maintaining appropriate pH levels in biological experiments and cell culture media.
- Aquariums and Aquaculture: Ensuring suitable pH levels for aquatic organisms.
Conclusion
A pH meter is an indispensable tool in scientific and industrial applications for accurately determining the acidity or alkalinity of solutions. Despite its limitations, advancements in technology have made pH meters more robust, user-friendly, and versatile. Proper maintenance and calibration ensure their reliable performance across a wide range of applications.