A Zinc-Air Battery is a type of metal-air battery that utilizes oxygen from the air and zinc metal as the primary reactants to generate electricity. These batteries are known for their high energy density, lightweight design, and eco-friendly nature, making them ideal for specific applications like hearing aids, electric vehicles, and backup power systems.
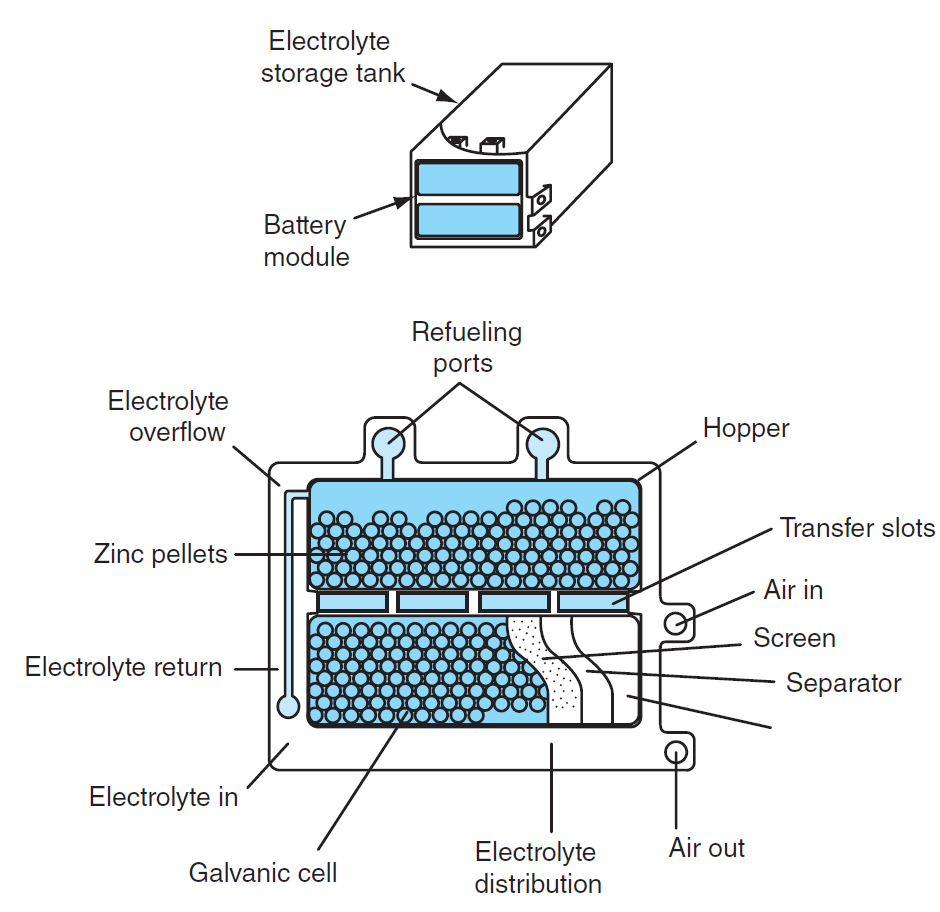
Construction of Zinc Air Battery
The basic components of a zinc-air battery are as follows:
- Electrolyte Storage Tank: Holds the electrolyte, which is usually an aqueous solution of potassium hydroxide (KOH) or sodium hydroxide (NaOH). This acts as the medium for ion transport between electrodes.
- Battery Module: The central part containing:
- Hopper: Houses zinc pellets or powder used as the anode material.
- Refueling Ports: For replenishing the zinc or electrolyte.
- Electrolyte Overflow System: Manages excess electrolyte flow.
- Electrolyte Return: Returns used electrolyte for recirculation.
- Electrolyte Distribution System: Ensures even distribution of the electrolyte across the electrodes.
- Galvanic Cell: The cell where the electrochemical reaction takes place, consisting of:
- Zinc Pellets (Anode): Zinc reacts with the electrolyte to release electrons.
- Air Cathode: A porous structure that allows oxygen from the air to enter the cell and participate in the reaction.
- Separator: Prevents direct contact between the zinc anode and air cathode, avoiding short circuits while permitting ionic flow.
- Air Management System:
- Air Inlet: Supplies oxygen to the cathode.
- Air Outlet: Releases excess air and reaction by-products.
Working Principle of Zinc Air Battery
The zinc-air battery operates based on a redox reaction between zinc and oxygen. The main steps are:
- Oxygen Reduction (Cathode Reaction): Oxygen from the air diffuses into the air cathode. It reacts with water in the electrolyte to form hydroxide ions.
- Zinc Oxidation (Anode Reaction): Zinc reacts with the hydroxide ions in the electrolyte, forming zincate ions and releasing electrons.
- Overall Reaction: The zinc hydroxide eventually decomposes, releasing water and allowing the process to continue.
The flow of electrons from the zinc anode to the air cathode through an external circuit generates electrical power.
Advantages of Zinc Air Battery
- High Energy Density: Zinc-air batteries offer higher energy per unit weight compared to most traditional batteries.
- Lightweight: The reliance on atmospheric oxygen instead of a built-in oxidizer reduces weight.
- Environmentally Friendly: Made from abundant and non-toxic materials like zinc and water-based electrolytes.
- Cost-Effective: Zinc is inexpensive and widely available.
- Long Shelf Life: When not in use, zinc-air batteries retain their charge for extended periods.
- Refuelable: Zinc and electrolyte can be replenished in some designs, extending their operational life.
Disadvantages of Zinc Air Battery
- Limited Power Density: These batteries are better suited for low-drain applications.
- Air Management: Requires efficient air circulation for oxygen intake and by-product removal.
- Water Loss: Electrolyte evaporation may occur, requiring regular maintenance.
- Non-Rechargeable (Primary Cells): Most zinc-air batteries are non-rechargeable; however, secondary versions are under development.
- Slow Reactivation: Batteries take time to activate after being exposed to air.
- Degradation: Exposure to CO₂ in the air can form carbonates in the electrolyte, reducing efficiency.
Applications of Zinc Air Battery
- Hearing Aids: Due to their compact size and lightweight nature.
- Electric Vehicles (EVs): High energy density makes them suitable for EVs with long-range requirements.
- Backup Power Systems: For grid-scale energy storage and emergency lighting.
- Military Devices: Portable and lightweight energy sources for soldiers.
- Renewable Energy Integration: Used in conjunction with solar and wind systems for energy storage.
- Consumer Electronics: Potential future use in portable devices like laptops and smartphones.
Conclusion
Zinc-air batteries are a promising technology for applications demanding lightweight, high-capacity energy storage. While challenges like electrolyte management and CO₂ sensitivity remain, advancements in rechargeable versions and better air management systems can pave the way for broader adoption.