Electroplating is the process in which a layer of some metal is deposited for decorative or protective purposes on the articles of other base metal by electrolysis.
Equipments for Electroplating
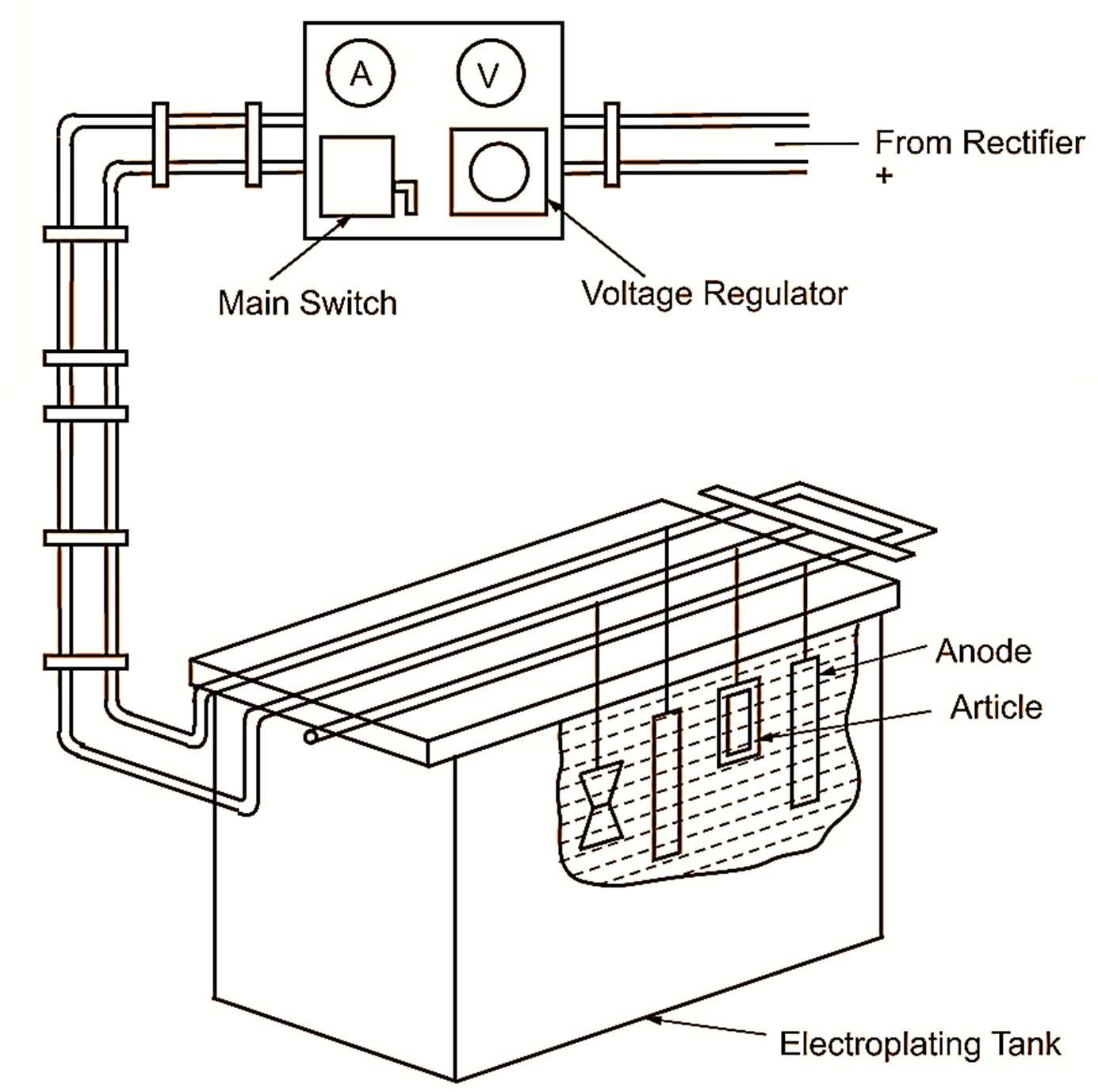
Figure 1: Electroplating.
Fig. 1 shows a small electroplating plant with the following equipments.
Electroplating Tank : For the solutions employed in electroplating, chemically resistant containers are generally necessary. Welded steel tanks suitably lined are used for the majority of electroplating processes. Wooden, R.C.C., fibre glass, stainless steel and enamelled iron tanks are also in use. The lining materials include lead, rubber, P.V.C. etc. The tank is filled with suitable electrolyte and has arrangement for hanging anodes and articles to be plated which normally form cathode. Apart from the electrolyte, some additional reagents like glue, gum, sugar, etc. are also added to obtain good results.
Power Supply Unit : For electroplating, direct current ranging from 50 to 1000 amperes or even more at 3 to 24 volts is required. Static rectifiers are normally employed for this purpose.
General Procedure for Electroplating
Before electroplating, the object must be perfectly clean, free from dirt, paint, scales, rust, grease, oil, etc.
- Cleaning is carried out using solvents like paraffin, hot alkaline cleaners (e.g. caustic potash) and acids.
- For smooth surface, mechanical operations like grinding, polishing are also carried out if necessary.
The object is then dipped in the bath for electroplating. The actual process of deposition of metal on the object forming the cathode by electrolysis has already been discussed previously. The metal to be deposited is supplied either by the anode of that metal or the electrolyte itself. The cathode (the article to be plated) is surrounded by the set of anodes or it is rotated slowly to ensure an even deposit all over. As a general rule, slower the deposition, the harder and more adherent it is. Hence, small currents are employed. The concentration and temperature of the electrolyte are other vital factors which affect the quality of the plating. Plating time depends on the mass of metal to be deposited. After plating, the article is washed and dried. In the cases of nickel, silver and chromium plating, the plated article is finally buffed with polishing mop rotated at high speed, for a bright metallic finish.
Applications of Electroplating
Gold or silver plating is many times done for decorative or ornamental purposes. The cast iron and steel parts are normally coated with zinc, nickel or chromium to prevent atmospheric corrosion. Nickel or chromium plating also gives shining white surface. Copper plating is mostly used on iron articles to prevent rusting or as preliminary coating for nickel or silver plating.