Metallic conductors carry an electric current without any change in their physical and chemical state, except, perhaps, a rise in temperature. However, there are some liquids which can conduct an electric current, but the passage of current through them is always accompanied by a chemical change. Such liquids are known as electrolytes. Various chemical compounds or solutions of compounds serve as electrolytes. e.g. electrolytes can be formed when certain salts, acids or alkalies are dissolved in water.
Electrolysis Process
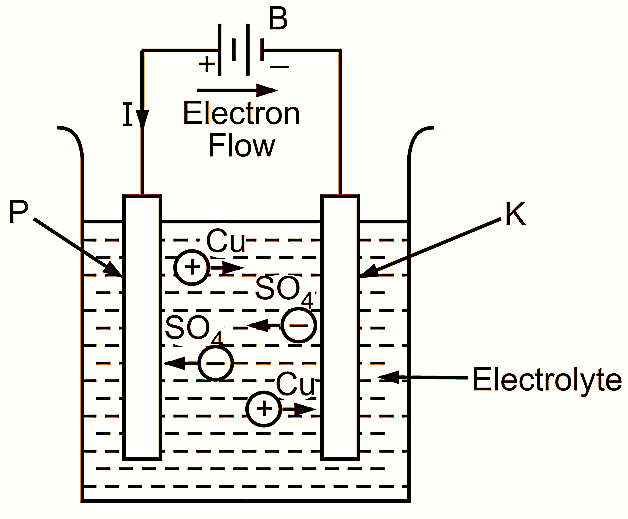
Figure 1: Electrolysis.
To understand the process of electrolysis, consider the solution of copper sulphate (CuSO4) in water. As the moment, copper sulphate (CuSO4) is dissolved in water, its molecules dissociate into positively charged copper (Cu) ions and negatively charged sulphate (SO4) ions. The ions thus formed start wandering freely in the solution.
Now, if two electrodes (P and K) are immersed in the solution and a battery (B) is connected as shown in Fig. 1, let us see the result. As soon as the potential difference is applied across the electrodes with the help of the battery, the haphazard movement of the ions becomes a directed movement. The positive copper ions are attracted to the negative electrode (K) called cathode, and the negative sulphate ions to the positive electrode (P) called anode.
On reaching the respective electrodes, they give up their charges and become ordinary molecules. Thus the copper ions become ordinary molecules of copper, which are deposited on the cathode. The sulphate ions also become ordinary sulphate molecules at the anode.
As the uncharged sulphate ion is incapable of separate existence, it immediately reacts with water (H2O) forming sulphuric acid (H2SO4) and liberating oxygen gas (O2) at the anode. The chemical reaction can be stated as follows :
\[2S{{O}_{4}}=2{{H}_{2}}O=2{{H}_{2}}S{{O}_{4}}+{{O}_{2}}\uparrow \]
If the anode is a suitable copper plate, the sulphate ion acts on it to form copper sulphate as given below :
\[S{{O}_{4}}=Cu=CuS{{O}_{4}}\]
This newly formed copper sulphate goes into solution and thus the density of the electrolyte is maintained constant. The net effect is that the copper from the anode is transferred to the cathode. The above process involving chemical decomposition of the electrolyte due to passage of current is known as electrolysis. It should be noted that the conduction of current in electrolyte is due to oppositely directed movements of positive and negative ions and it is always accompanied by a chemical change.
Faraday’s Laws of Electrolysis
In 1834, Faraday formulated following two basic laws based on his experimental work on electrolysis.
First Law
It states that the weight of a substance liberated from an electrolyte in a given time during the process of electrolysis is directly proportional to the quantity of electricity which has assed through the electrode.
Thus, if
W = Weight of the substance liberated in kg
Q = Quantity of electricity in coulombs
= Current (I) in amperes × Time (t) in seconds. Then, according to this law,
\[W\propto Q\propto I.t\]
Or,
\[W=Z.I.t\]
Where, Z is a constant called the electrochemical equivalent.
It is defined as the mass of the particular substance liberated in unit time by unit current and its unit is the kilogram per coulomb.
Second Law
It states that if the same current flows through several electrolytes, the weights of the substances liberated from each are proportional to the chemical equivalents of these substances.
The chemical equivalent of a substance is the weight of the substance which can displace or combine with unit weight of the substance in a chemical reaction.
With the above definition, the chemical equivalent of hydrogen itself will be obviously one.
Extraction and Electro refining of Metals
The metals like aluminium, copper, magnesium, sodium, zinc, etc. can be extracted from their ores and refined by electrolytic processes.
In electrolytic extraction process, the ore is either melted or treated with strong acid. The pure metal is then obtained by the electrolysis of this solution. The metals obtained by electrolytic extraction methods being 98 to 99 percent pure, these methods are always preferable in comparison with the other metallurgical processes available for the most of these metals. Sometimes, however, this electrolytic extraction cannot be carried out economically. Therefore, in such cases, only the refining is done electrolytically.
Copper is extracted by metallurgical as well as electrolytic processes. However, it is always refined electrolytic ally to its purest form as required by the electrical industries. For refining of metals, ingots of impure metal obtained from metallurgical process are used as anodes. They are placed in an electrolytic cell containing a suitable electrolyte. The current is then passed through the bath. Due to electrolysis, the pure metal gets deposited on the cathode made of pure metal.
Power Supply: Large amount of d.c. power is required for extraction and refining of metals. Voltage per electrolytic cell is only about 10 V. However, a number of such cells can be connected in series, so that the voltage required is 500 to 800 volts, with currents upto several thousand amperes. Special heavy current motor-generator sets, rotary convertors or mercury arc rectifiers are used for conversion of a.c. to d.c.