A fuel cell is an electrochemical device that converts chemical energy from a fuel (typically hydrogen) and an oxidizing agent (such as oxygen) directly into electrical energy, with water and heat as by-products. Unlike traditional combustion-based power generation, fuel cells do not involve burning fuel, resulting in highly efficient and environmentally friendly energy production.
Construction of a Fuel Cell
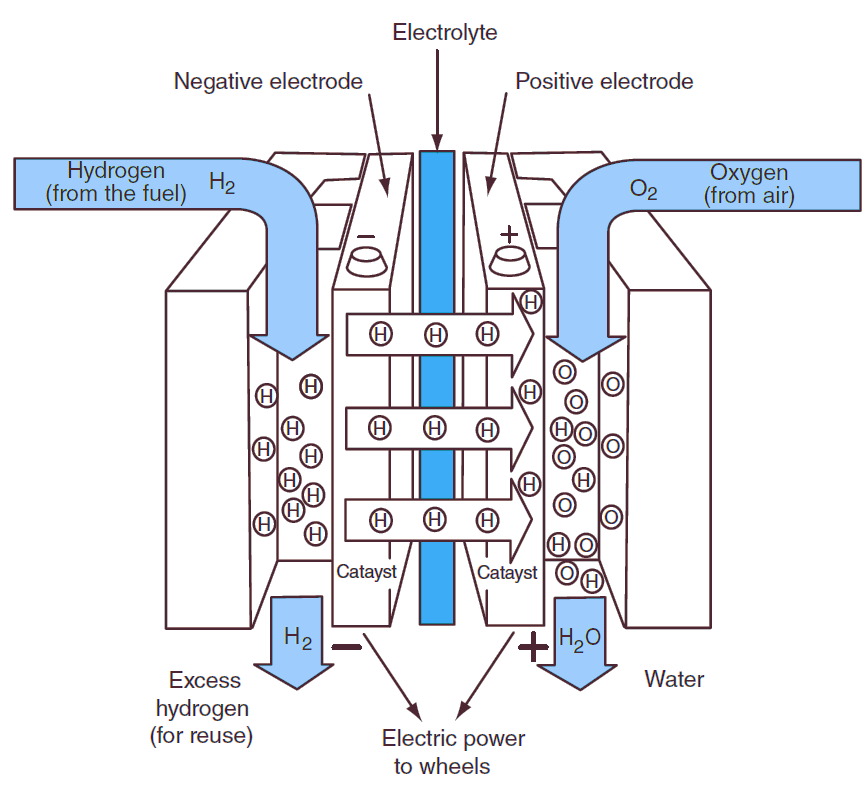
Figure 1: Fuel Cell.
A fuel cell consists of several essential components that work together to produce electricity:
- Anode (Negative Electrode): This is where the fuel, typically hydrogen, is supplied. The hydrogen molecules are split into protons (H+) and electrons (e⁻) at the anode, often facilitated by a catalyst.
- Cathode (Positive Electrode): Oxygen from the air reacts with the protons and electrons here, producing water as a by-product.
- Electrolyte: The electrolyte allows only the movement of ions (protons) between the electrodes while preventing electrons from passing through. It can be a liquid, solid polymer, or ceramic material, depending on the type of fuel cell.
- Catalyst: A catalyst, such as platinum, is used to accelerate the chemical reactions at the anode and cathode. It reduces the energy barrier for the reaction without being consumed.
- Fuel: Hydrogen is the most commonly used fuel, but other fuels like methanol or natural gas can also be utilized after reformation into hydrogen.
- Oxidant: Oxygen (usually from air) acts as the oxidizing agent.
- Bipolar Plates: These plates distribute the reactants (fuel and oxidant) evenly across the electrodes, collect the produced electricity, and remove by-products like water and heat.
Working of a Fuel Cell
- Introduction of Reactants: Hydrogen is fed into the anode, and oxygen (or air) is supplied to the cathode.
- Electrochemical Reaction at the Anode: t the anode, the hydrogen molecules are split into protons (H+) and electrons (e⁻) by the catalyst:
- Movement of Protons: The electrolyte allows the protons to pass through it from the anode to the cathode.
- Flow of Electrons: The electrons travel through an external circuit, generating an electric current that can power a device or vehicle.
- Reaction at the Cathode: At the cathode, the protons, electrons, and oxygen react to form water:
- By-Products: The by-products of the process are water and heat.
Types of Fuel Cells
| Type | Electrolyte | Operating Temperature | Applications |
|---|---|---|---|
| Proton Exchange Membrane Fuel Cell (PEMFC) | Solid Polymer | 50-100°C | Vehicles, Portable Devices |
| Solid Oxide Fuel Cell (SOFC) | Ceramic | 500-1,000°C | Stationary Power, Industrial Use |
| Alkaline Fuel Cell (AFC) | Potassium Hydroxide | 60-90°C | Spacecraft, Military Applications |
| Phosphoric Acid Fuel Cell (PAFC) | Phosphoric Acid | 150-200°C | Hospitals, Data Centers |
| Molten Carbonate Fuel Cell (MCFC) | Molten Carbonate | 600-700°C | Electric Utilities, Industrial Use |
Advantages of Fuel Cells
- High Efficiency: Fuel cells convert chemical energy directly into electrical energy, reducing energy losses associated with combustion.
- Environmental Benefits: They emit only water vapor and heat, making them a clean alternative to fossil fuel-based technologies.
- Scalability: Fuel cells can be used in various applications, from small portable devices to large power plants.
- Quiet Operation: Unlike internal combustion engines, fuel cells operate silently.
- Renewable Potential: When hydrogen is produced from renewable sources, fuel cells become a sustainable energy solution.
- Energy Security: Fuel cells reduce dependency on fossil fuels, promoting energy diversification.
Disadvantages of Fuel Cells
- High Initial Cost: The use of expensive materials like platinum for catalysts increases the cost of fuel cells.
- Hydrogen Infrastructure Challenges: Limited availability of hydrogen fueling stations and the difficulty of hydrogen storage and transportation hinder widespread adoption.
- Durability and Reliability: The performance of fuel cells can degrade over time, especially under variable operating conditions.
- Complexity of Technology: Fuel cell systems involve sophisticated designs and control mechanisms.
- Energy Loss in Hydrogen Production: Producing hydrogen from water or other fuels often requires significant energy input, reducing overall efficiency.
Applications of Fuel Cells
- Transportation: Fuel cells are used in hydrogen-powered vehicles, such as cars, buses, trucks, and trains. Examples include the Toyota Mirai and Hyundai Nexo.
- Stationary Power Generation: Fuel cells provide backup power and primary electricity for buildings, hospitals, and data centers.
- Portable Power: Small fuel cells are used to power portable devices like laptops, smartphones, and military equipment.
- Maritime Applications: Hydrogen fuel cells power submarines, boats, and ships.
- Industrial Applications: They are employed in forklifts, cranes, and other machinery for indoor and outdoor use.
Conclusion
Fuel cells represent a promising clean energy technology with the potential to transform the way we produce and consume energy. While there are challenges to overcome, such as cost and infrastructure development, ongoing research and advancements are paving the way for broader adoption. Fuel cells are expected to play a vital role in achieving a sustainable and low-carbon energy future.